What is the NIMBLE Study?

What is the NIMBLE Study?
Why are we doing this study?
If you are diagnosed with generalized myasthenia gravis (gMG), please know that you are not alone in your medical journey. gMG is a rare autoimmune disorder caused by autoantibodies produced by the immune system attacking the body’s own cells. These autoantibodies activate a pathway in the immune system called the complement pathway that leads to disruption of the communication between nerves and muscles, so that the muscle cannot function normally.
gMG affects between 50 and 200 people per million worldwide.
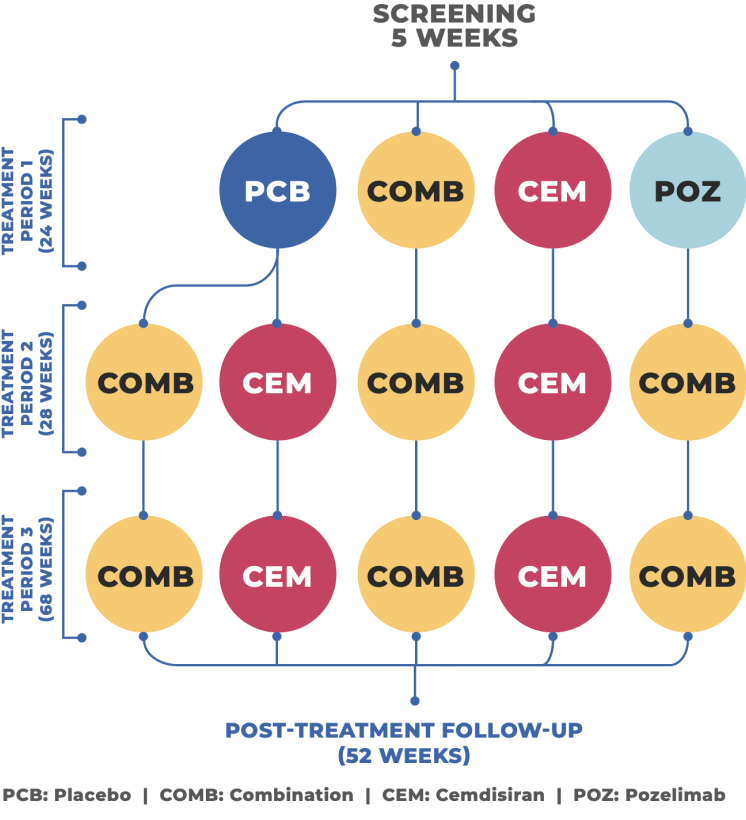
What study drugs will I receive?
Investigational drug 1 (pozelimab):
A monoclonal antibody that blocks your complement component 5 (C5). In people with gMG, the action of C5 damages the communication between the nerves and muscles, which disturbs normal muscle contraction.
What can I expect if I participate?
Taking part in a clinical study is a commitment. The study team will share information on what to expect, as well as your roles and responsibilities during the study.
What if I no longer want to participate in this clinical trial?
Study participation is completely voluntary (your choice). You can change your mind and leave the study at any time. You do not need to give a reason. If you leave the study, your medical care outside of the study will not be affected by your decision.
How can I take part?
What else should I know?
There is no guarantee that you will receive a medical benefit from taking part in this clinical study. The study drug may cause side effects or affect your health in an unknown way. However, your safety is our top priority and your health will be closely monitored throughout this study.
Participants in clinical studies are essential to developing treatments and increasing our medical understanding of myasthenia gravis. The information from this clinical study may benefit others in the future.
Investigational drug 1 (pozelimab):
A monoclonal antibody that blocks your complement component 5 (C5). In people with gMG, the action of C5 damages the communication between the nerves and muscles, which disturbs normal muscle contraction.